医疗器械单一审核方案(MDSAP)
医疗器械单一审核方案(MDSAP)


什么是MDSAP审核?
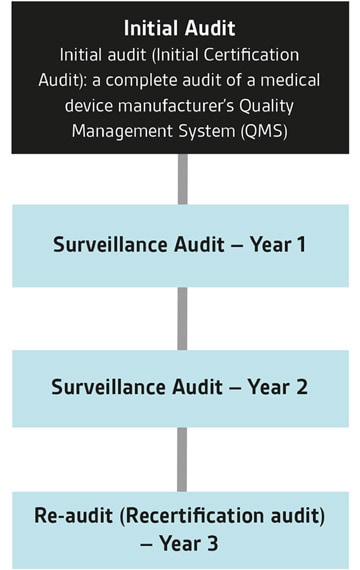
医疗器械单一审核方案(Medical Device Single Audit Program ,缩写为MDSAP)是由国际医疗器械法规论坛(IMDRF)发起,以ISO 13485为基础,结合五个参与国(美国、加拿大、巴西、澳大利亚和日本)的法规要求,由认可的审核机构进行一次审核满足多国法规要求的方案。
BSI可以提供MDSAP + ISO 13485 +UKCA+ CE的整合审核方案。
MDSAP审核由具备授权资质的审核机构(如BSI)进行。BSI已为大量世界领先的医疗器械制造商颁发了MDSAP证书。
MDSAP涵盖哪些地域和监管机构?
如果医疗器械制造商的产品出口涉及如下国家,可考虑进行MDSAP认证。加入MDSAP的五家监管机构已就如何执行和使用MDSAP发表如下声明:
MDSAP指南
全面了解MDSAP
-
MDSAP认证服务手册 >
MDSAP is a way that medical device manufacturers can be audited once for compliance with the standard and regulatory requirements of up to five different medical device markets
-
MDSAP七大审核流程的分析指南 >
Learn more about our training courses, available dates and booking options
-

MDSAP网络研讨会 >
We offer a wide range of free and live webinars hosted by BSI Technical Specialists addressing key topics that affect your business including legislation, risk, and regulatory changes.
-

医疗器械白皮书 >
Our white papers will help you understand how to perform better, reduce risk and make excellence a habit in your organization.