An ineffective audit can mean severe consequences; resulting in process failure, patient dissatisfaction and regulatory noncompliance. Optimize your auditing skills with the internationally recognized ISO I3485:2016 and boost your internal audit capabilities. Gain confidence in planning and performing an effective audit, as well as reporting and taking corrective action where necessary.
This course is intended for medical device quality professionals wishing to build on their current knowledge of ISO 13485:2016 and evaluate the effectiveness of their QMS. It teaches the principles and practices of effective audits in accordance with ISO 13485:2016 and ISO 19011:2018.
Delegates learn how to audit the processes of an ISO 13485:2016 Quality Management System (QMS). This course provides guidance and practical experience in planning, executing, reporting and audit follow-up of an internal audit when monitoring the effectiveness and conformity of a ISO 13485:2016 compliant QMS
Online training courses: Connected Learning Live
You can attend this training course classroom based and online. Do you prefer the convenience of an online training course? View here for the available dates or read more about Connected Learning Live.
Lees deze pagina in het Nederlands en bekijk beschikbare data van de Nederlandstalige trainingen
This course forms part of our Auditor Qualification programme. To find out more, please visit our training auditor qualification page here.
Gain a Certificate of Achievement for this course
You can now qualify for a Certificate of Achievement, by passing the assessment requirements, including an end-of-course online exam, you’ll improve your professional profile and be able to:
- Provide evidence of your learning
- Demonstrate your competence
We will email your exam log-in details when you’ve finished the course. The exam is done online which means you can choose when and where to complete it. You are strongly advised to choose a time and a place where you will not be disturbed, and where you have access to a reliable internet connection. The exam takes approximately 80 minutes, is comprised with 40 multiple choice questions and you have up to 30 days to complete it – including one opportunity for a re-take.
Upon successful completion of this exam you will be awarded a Certificate of Achievement alongside your Certificate of Attendance. If, however, you decide not to complete the exam, you will still be awarded with a Certificate of Attendance.
Please talk to a member of our training team on +31 (0)20 346 0780 or via training.nl@bsigroup.com if you have any questions in regards to the online exam and your training course.
ISO 13485:2016 learning path and combination discount
The ISO 13485:2016 Internal Auditor learning path is modular. You can follow the training in all combinations. We recommend the following order:
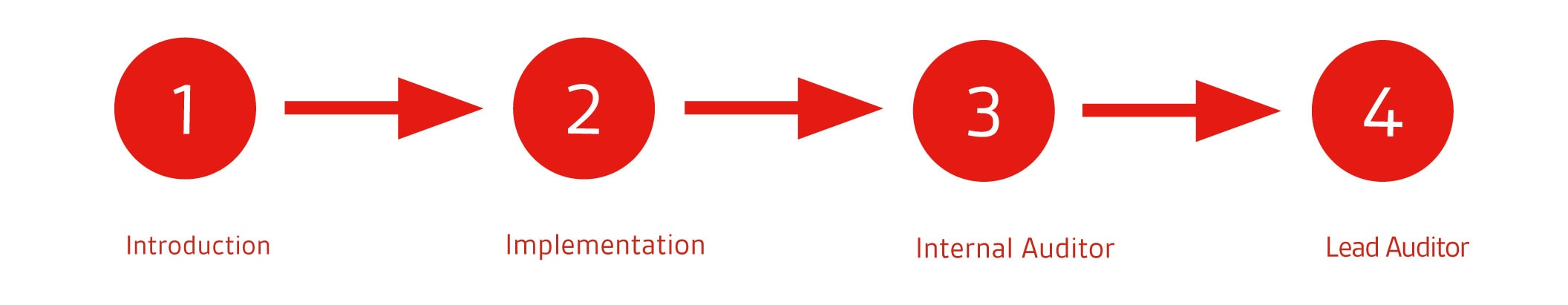
Besides this introduction course, are the following training courses also part of the ISO 13485 learning path:
1. ISO 13485 Introduction
2. ISO 13485 Implementation
3. ISO 13485 Internal Auditor
4. ISO 13485 Lead Auditor
Attending several training courses of a learning path is associated with special discounts.
| Training course |
| Combination training 1 + 2 |
| Combination training 1 + 3 |
| Combination training 1 + 4 |
| Combination training 1 + 2 + 3 |
| Combination training 1 + 2 + 3 + 4 |
In-house training course
If more employees need to understand, implement, audit, your management system, training can be delivered at your location. Based on your learning needs we could provide a customized in-house training course for your team. For more information about the learning path or in-house training courses, please contact our training team via +31 20 346 0780 or send an email to training.nl@bsigroup.com.