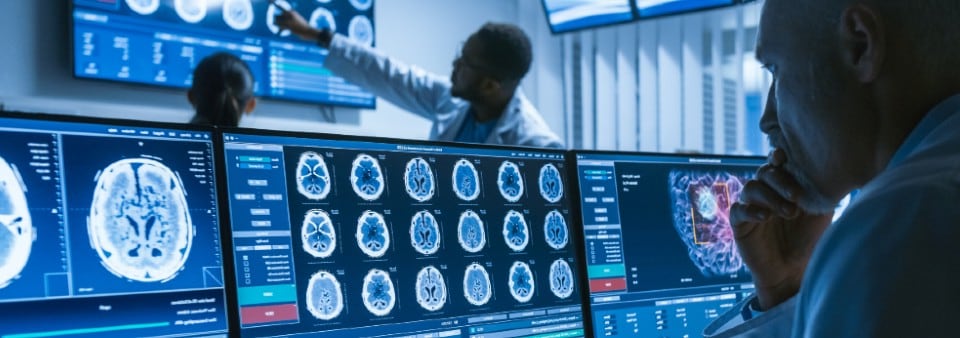
Is my software a medical device?
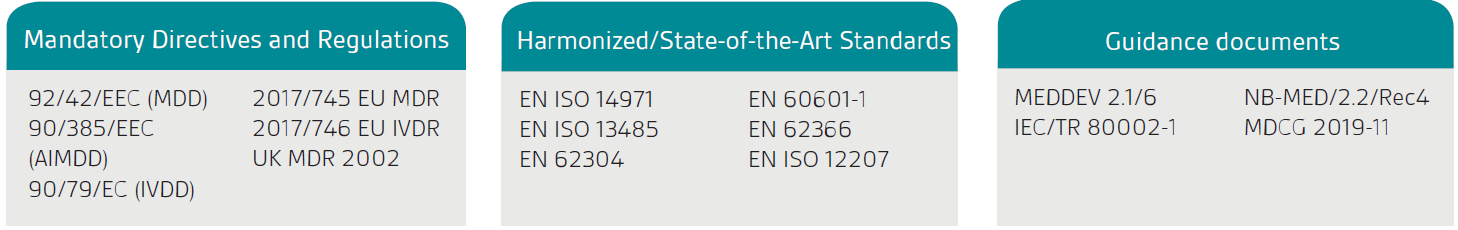
The first stage is to confirm your product or service is legally classified as an SaMD; the product must first have a stated intended purpose that is medical as defined by the Medical Device Directives and Regulation in the EU and the UK Medical Devices Regulation (UK MDR) 2002.
The European Commission’s guidance, MEDDEV 2.1/6, is only applicable to standalone software.
As indicated in the EU MDD/MDR and UK MDR, standalone software which has a medical purpose is considered to be an active medical device. Classification depends on the risk to the patient and users. To classify your software fully, you will need to review the relevant classification rules.