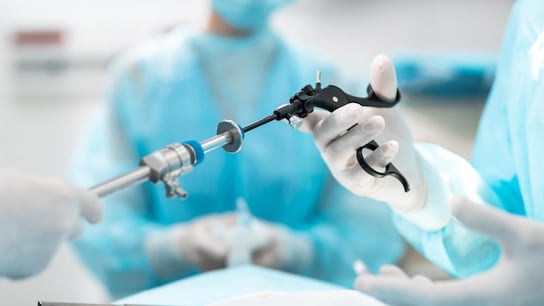
Compliance with the state of art standards is the preferred route to demonstrate compliance with the applicable General Safety and Performance Requirements of the MDR or with the Essential Requirements of the UK Regulation. As few standards are currently harmonized to the regulations, when being assessed against the Regulation, understanding and meeting the requirements of EN 60601 is crucial. EN 60601 represents a state-of-the-art standard and can therefore be applied to show conformity with the applicable Regulations requirements.
- Australia - English
- Austria - Deutsch
- Belgium - Nederlands
- Brazil - Português
- Canada - Français
- Chile - Español
- China - 简体中文
- Colombia - Español
- Costa Rica - Español
- Czech Republic - English
- France - Français
- Germany - Deutsch
- Hong Kong (SAR China) - English
- India - English
- Indonesia - English
- Ireland - English
- Israel - English
- Italy - Italiano
- Japan - 日本語
- Korea - 한국어
- Malaysia - English
- MEA - عربي
- MEA - English
- Mexico - Español
- Mongolia - English
- Netherlands - English
- Netherlands - Nederlands
- New Zealand - English
- Peru - Español
- Philippines - English
- Poland - Polski
- Singapore - English
- South Africa - English
- Spain - Español
- Sweden - English
- Switzerland - Deutsch
- Taiwan - 繁體中文
- Thailand - ไทย
- Turkey - Türkçe
- United Kingdom - English
- United States - English
- Vietnam - English
- Vietnam - Tiếng Việt
Suggested region and language based on your location
Your current region and language